Novak Laboratory
About the Lab
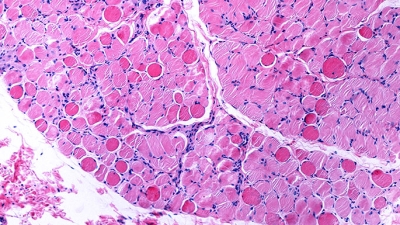
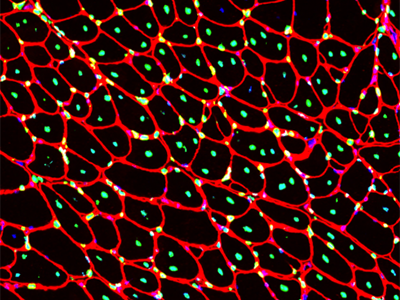
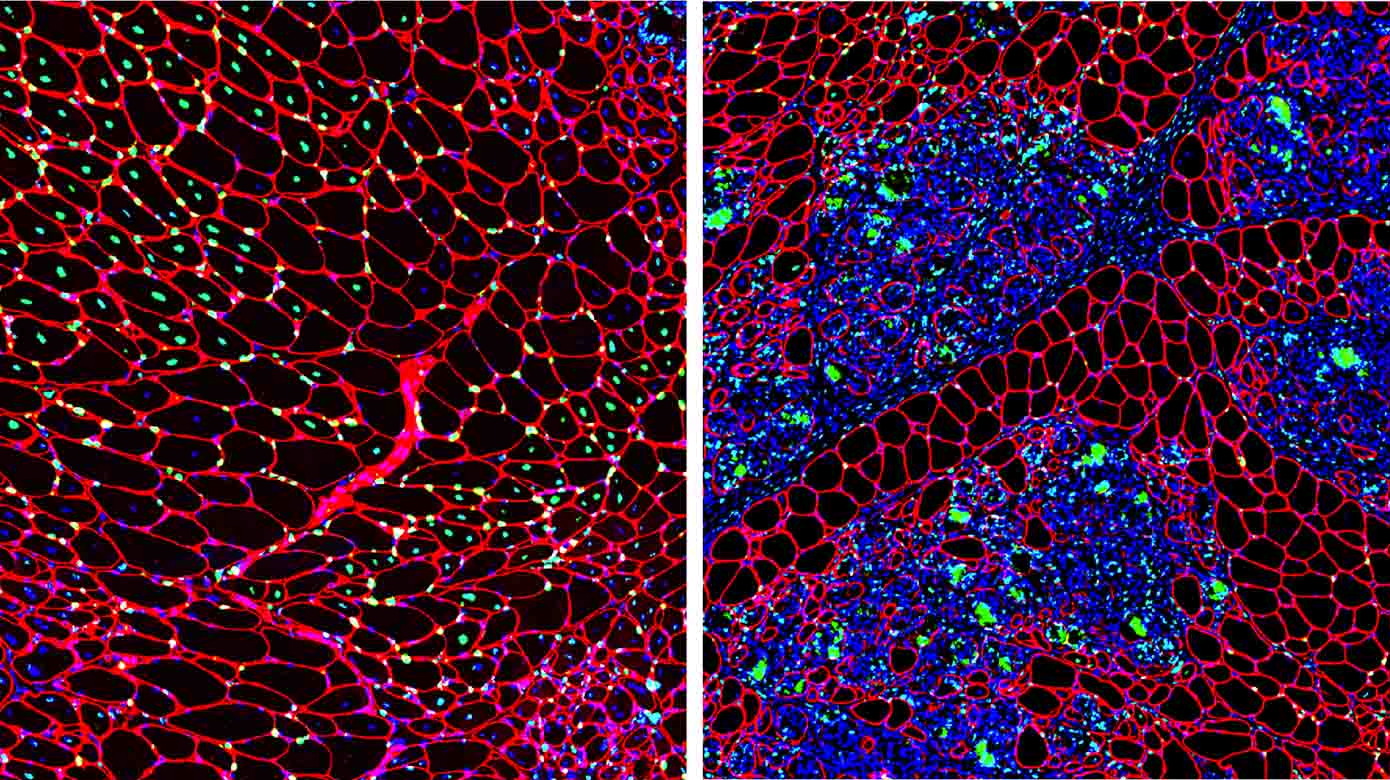
Muscular dystrophies are genetic disorders characterized by muscle regenerative deficits, extensive muscle loss and fibrosis. One such disease, Duchenne muscular dystrophy (DMD), is a severe and relentlessly progressive myopathy that results from mutations in the X-linked DMD gene that disrupt the mRNA reading frame and prevent translation of the muscle structural protein, dystrophin. In DMD, the lack of dystrophin leads to chronic muscle degeneration, inflammation and fibrosis, resulting in a loss of muscle structural integrity and myofiber death. A promising genetic therapeutic strategy for DMD and other neuromuscular diseases is 'exon skipping' through the use of antisense oligonucleotides (AOs). This class of drug allows for ‘exon skipping’ of the mutated exons in the patient’s dystrophin gene to restore a functional, truncated dystrophin protein, and is currently the only approved genetic therapy for the treatment of DMD. A major focus of our research investigates how the complex pathological processes in DMD and muscular dystrophies modulate the delivery and therapeutic efficacy of antisense chemistries and gene therapies to develop novel delivery strategies to improve the pharmacokinetics and efficacy of these therapeutic agents for muscle diseases.
-
Lab Focus Areas
Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), Facioscapulohumeral muscular dystrophy (FSHD)
Skeletal and cardiac muscle disease pathogenesis Therapeutic targets for muscular dystrophies
Drug delivery strategies for muscle disease
Muscle satellite cell dynamics during growth, aging and disease -
Partnership
George Washington University School of Medicine and Health Sciences
-
Contact
James Novak, PhD Principal Investigator [email protected] 202-476-6135
Highlights from the Lab
-
Internship Opportunities
Our laboratory is committed to training and mentoring the next generation of young scientists.
-
News
Experimental model mimics early-stage myogenic deficit in boys with DMD.
Featured Publications
-
TGF-beta-driven muscle degeneration and failed regeneration underlie disease onset in DMD mouse model
Mázala DA*, Novak JS*, Hogarth MW, Nearing M, Adusumalli P, Tully CB, Habib NF, Gordish-Dressman H, Chen YW, Jaiswal JK, Partridge TA *Denotes joint first-authorship PMID: 32213706 JCI Insight 5(6). PMID: 32213706 (2020) -
Morpholino-induced exon skipping stimulates cell-mediated and humoral responses to dystrophin in mdx mice
Vila MC*, Novak JS*, Hogarth MW, Klimek MB, Boehler J, Gordish-Dressman H, van den Anker J, Yokota T, Lu QL, Hoffman EP, Partridge TA, Nagaraju K. *Denotes joint first-authorship PMID: 30883742 Journal of Pathology 248(3): 339-351 (2019) -
The macrophage as a Trojan horse for antisense oligonucleotide delivery
Novak JS, Jaiswal JK, Partridge TA PMID: 29860876 Expert Opinion Therapeutic Targets 22:463-466 (2018) -
Shorter phosphorodiamidate morpholino antisense oligonucleotides (PMO-AONs) in higher doses may increase exon skipping efficacy in Duchenne muscular dystrophy patients
Akpulat U, Becker K, Partridge TA, Novak JS, Cirak S PMID: 30396145 Molecular Therapy 13: 534-542 (2018) -
Myoblasts and macrophages are required for therapeutic morpholino antisense oligonucleotide delivery to dystrophic muscle
Novak JS, Hogarth MW, Boehler JF, Nearing M, Vila MC, Heredia R, Fiorillo AA, Zhang A, Hathout Y, Hoffman EP, Jaiswal JK, Nagaraju K, Cirak S, Partridge TA PMID: 29038471 Nature Communications 8:941 (2017)