Jyoti Jaiswal, MSc, PhD, and James Novak, PhD, along with their team member, Young Jae Moon, PhD, jointly developed a combination oligonucleotide therapy for treating muscular dystrophy. Dr. Jaiswal studies the biology of musculoskeletal and respiratory diseases, and use these insights to develop molecular and genetic therapies to treat these conditions. Dr. Novak was trained as a molecular and cellular pharmacologist and is focused on the development of therapeutic strategies for muscular dystrophies that target the underlying genetic cause of the disease and ameliorate disease pathogenesis.
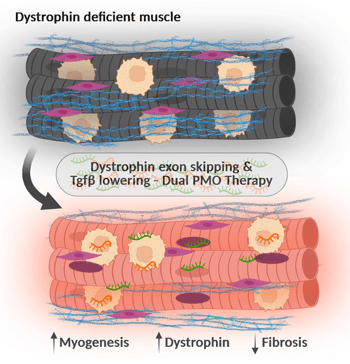
The novel approach developed here uses a combination oligonucleotide therapy to treat musculoskeletal diseases involving elevated TGF-β activity, with a particular focus on Duchenne muscular dystrophy (DMD). Muscle wasting and fibrosis are significant muscle pathologies and a hindrance to effective exon skipping therapies approved for DMD. This novel approach uses combinatorial antisense oligonucleotide therapy to inhibit TGF-β activity to improve myogenesis, reduce fibrosis and enhance dystrophin production. This approach overcomes the limitations of current DMD therapies using PMO (Phosphorodiamidate Morpholino Oligomer)-based antisense oligonucleotide therapy due to poor delivery of PMO-based antisense oligonucleotides. The novel combination oligonucleotide therapy that includes PMOs that not only include dystrophin exon skipping PMOs (DPMO), but also PMOs that lower TGF-β, addresses underlying pathological barriers to effective delivery of dystrophin-targeted exon skipping antisense oligonucleotides, specifically poor regeneration and excessive fibrosis, to enhance dystrophin restoration in skeletal and cardiac muscles.
The technology is a TGF-β-lowering antisense oligonucleotide (AO) therapy (TPMO) that goes beyond treating DMD to other related disorders associated with elevated TGF-β activity.
Problems addressed
- Impaired muscle regeneration and excessive fibrosis due to TGF-β overactivity.
- Limited efficacy of current PMOs in severe DMD models and DMD patients.
- Poor dystrophin restoration in myofibers and cardiomyocytes following PMO antisense oligonucleotide therapy.
Key features and advantages
- TPMOs inhibit TGF-β signaling to enhance muscle regeneration and lower fibrosis.
- Dual therapy (TPMO + DPMO) restores significant dystrophin level and muscle function.
- Enables PMO-based AO delivery to cardiomyocytes.
- Targets TGF-β production in damaged muscle regions, reducing off-target effects.
- Safe in chronic use, with no adverse behavioral or organ effects.
- Restores dystrophin-associated protein complex and muscle fiber integrity.
This innovative approach is different from existing solutions and fills critical gaps.
Critical gaps
- Dystrophin exon skipping PMOs used in DMD are limited by their poor delivery resulting in low dystrophin restoration.
- There is lack of approaches for safe and efficacious downregulation of TGF-β.
Key differentiators
- First combinatorial PMO AO therapy targeting both TGF-β and dystrophin.
- Allows monotherapy for conditions caused by elevated TGF-β activity.
- Restores dystrophin expression in cardiomyocytes - unachievable with existing dystrophin exon-skipping AO monotherapies.
- Broadly applicable across DMD mutations due to the universal role of TGF-β in DMD pathogenesis.
- Demonstrates synergy: improved exon skipping, dystrophin protein restoration and muscle function.
This technology has strong potential to transform DMD care
- Clinical Benefits: Improved muscle strength, regeneration and function; significant dystrophin restoration in skeletal muscles and improved cardiac muscle dystrophin restoration.
- Applicability: Effective across DMD genotypes; can serve as monotherapy or in combination with existing exon-skipping AO therapies.
- Safety: Well-tolerated in chronic use, with no observed toxicity.
- Commercial Potential: Addresses major unmet needs in DMD therapy, with broad market scope and application in severe cases where current therapies fail.
Who should partner with us?
Dr. Jyoti Jaiswal and Dr. James Novak are interested in partnering with biotech companies, gene therapy focused startups and pharmaceutical companies which focus on DMD, neuromuscular diseases and rare genetic diseases.
Funding and future outlook
This project has been funded by a Department of Defense (DoD) grant award and pilot funding through the Foundation to Eradicate Duchenne.
The outlook is promising, as the therapy could:
- Enhance outcomes of current dystrophin exon skipping PMO AO therapies across the DMD spectrum.
- Works as a standalone treatment to target heightened TGF-β activity.
- Improves dystrophin restoration in skeletal and cardiac muscles.
- Addresses key barriers in DMD care with a safe, effective and scalable approach.
This study was published in Molecular Therapy Nucleic Acids, Volume 36, Issue 3, 102665, September 09, 2025 as A combinatorial oligonucleotide therapy to improve dystrophin restoration and dystrophin-deficient muscle health, Moon, Young Jae et al.