Scientists and clinicians have pioneered a personalized approach to cancer treatment at the Children’s National Hospital Sheikh Zayed Institute for Pediatric Surgical Innovation (SZI). A bold new frontier in pediatric cancer immunotherapy has arrived which harnesses the body’s own immune system by training a patient’s own blood cells to recognize and destroy deadly solid tumors like neuroblastoma.
The Innovation for Pediatric Cancer Immunotherapy
The innovation, trained autologous T-Cells, is the result of years of translational research led by Anthony Sandler, MD, at Children’s National Hospital. In a groundbreaking study just published in Frontiers in Immunology, researchers introduced a novel, patient-specific therapy that teaches the immune system how to fight cancer cells using the patient’s own T-cells derived from peripheral blood. This new technology has broad potential for treating multiple pediatric solid tumors, such as neuroblastoma, melanoma, astrocytoma, medulloblastoma and rhabdomyosarcoma.
Dr. Sandler is the senior vice president and surgeon-in-chief of the Joseph E. Robert, Jr., Center for Surgical Care at Children’s National Hospital. His research interests include tumor immunology, tumor vaccine therapy and surgical devices. Xiaofang Wu, MD, associate research professor of pediatrics, a co-inventor of this technology, has expertise and research interest in immunotherapies for pediatric solid tumors, MYC oncogene, whole tumor cell vaccines and mitigating immune-related adverse events. Dr. Sandler and his team understand the limitations of current therapy for childhood tumors and stive to find more effective and safer therapies for treating childhood cancer.
Here’s how it works:
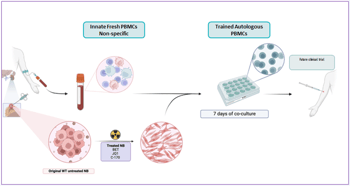
1. Tumor samples and blood are collected from the child during surgery.
2. Tumor cells are modified in the lab and treated with MYC inhibitors and STING pathway modulators to make them more visible to the immune system.
3. Patient blood cells (PBMCs, Peripheral Blood Mononuclear Cell) are then exposed to these modified tumor cells under controlled lab conditions. This “trains” the T-cells to recognize and kill the cancer, i.e. training T-cells outside the body and then reintroducing them.
4. The result is a personalized population of trained cytotoxic T-cells, capable of selectively targeting the child’s own tumor.
“This is a personalized immune response designed for each child—using their own blood cells, trained to destroy their own tumor,” said Dr. Sandler.
Why this matters: Neuroblastoma is one of the most common solid tumors in children and among the most difficult to treat. Standard chemotherapy and radiation, while often effective at shrinking tumors, can leave behind resistant cells that lead to relapse. In high-risk cases, survival rates can dip below 10%. Immunotherapy, especially T-cell-based therapy, has revolutionized treatment for blood cancers, but translating that success to solid tumors has been a challenge. This technology provides novel therapy by training T-cells outside the body and then reintroducing them. This novel approach bypasses the common pitfalls of previous cell therapies by avoiding issues of T-cell exhaustion, toxicity and lack of tumor infiltration. This breakthrough changes the game by making cancer visible to the immune system. The team’s innovation lies in how they make neuroblastoma cells “visible” to immune cells.
The features and advantages of this new technology include:
- Tumor cells are treated with MYC inhibitors (drugs that disrupt the cancer’s ability to hide).
- STING pathway inhibitors are used to suppress cancer’s defense mechanisms, paradoxically boosting immune recognition.
- These treated cells are then irradiated and used to “train” the immune system in the lab.
- Potent autologous T-cells capable of killing neuroblastoma cells in vitro and in preclinical mouse models without damaging healthy tissue or causing autoimmunity.
- There is no genetic manipulation of the cells.
Preclinical Success
The team has achieved preclinical success using this novel technology. When the mice was injected with trained T-cells, it completely rejected neuroblastoma tumors. No autoimmune reactions were observed, offering an important safety milestone. The trained immune cells displayed robust tumor-killing activity, even weeks after infusion. With this strong preclinical data, the team is now preparing for first-in-human studies. This technology represents a scalable, safe and personalized path forward for children battling aggressive cancers. The era of one-size-fits-all cancer treatment is ending. This new approach of utilizing ex vivo-trained autologous T-cell therapy signals a future where each child’s immune system becomes their most powerful weapon against cancer.
Who Should Partner with Us?
Dr. Sandler is actively seeking collaborators and commercial partners to accelerate clinical translation. Ideal collaborators include biotech companies in cell therapy or immune modulation, pediatric oncology networks looking to implement novel immunotherapies, investors and foundations focused on curative approaches for rare pediatric diseases and pharmaceutical partners seeking to license cutting-edge, non-CAR, T-cell technologies.
Related News
-

Predicting risk before it becomes harm in the pediatric ED
January 21, 2026 -

Collaboration and engineering in pediatric orthopaedics
January 12, 2026 -
AI tool shows promise for faster, more accurate pediatric tuberculosis detection
December 04, 2025