At the Children’s National Hospital Center for Cancer and Immunology Research (CCIR), our mission is to drive groundbreaking research to benefit children with cancer, infection, blood- and immune-related disorders. Jianhua Yang, PhD, in CCIR, whose research focuses on cancer biology, experimental cancer therapy and tumor immunology, is developing treatments for precision cancer therapy. He has developed novel therapies for both pediatric and adult cancers with a particular emphasis on neuroblastoma.
The current treatment options for neuroblastoma—a pediatric malignancy—remain unsatisfactory. Dr. Yang recognizes the unmet need for more precise and durable therapies and has become interested in targeting REarranged during Transfection (RET) as a therapeutic strategy. The RET proto-oncogene encodes a single-pass transmembrane receptor tyrosine kinase. During development, RET promotes proliferation and migration of neural crest-derived cells which is essential for the formation of the enteric nervous system and kidney. Receptor tyrosine kinase signaling in development is linked to multiple cancer types.
Developing safer and more targeted therapy
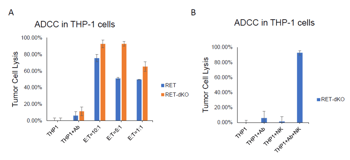
Dr. Yang and his team have developed a first-in-class monoclonal antibody that selectively targets wild-type RET, which is overexpressed in neuroblastoma, Ewing sarcoma, acute myeloid leukemia (AML), breast cancer and other tumor types. The antibody, i.e. anti-RET monoclonal antibody (RET-targeting antibody), is designed to bind RET on the surface of tumor cells, blocking its kinase signaling and mediating anti-tumor effects through immune effector mechanisms, including antibody-dependent cellular cytotoxicity (ADCC). This approach addresses a critical need for safer, more targeted cancer therapies—particularly in pediatric patients.
Differentiation from existing therapies by addressing unmet needs
Currently approved RET inhibitors, such as Selpercatinib and Pralsetinib, are small-molecule drugs that target RET mutations in selected cancer types. In contrast, this RET-targeting antibody represents a fundamentally different treatment approach by directly inhibiting tumor growth driven by wild-type RET and engaging the immune system to eliminate RET-expressing cancer cells. Monoclonal antibodies typically offer greater target specificity and improved safety profiles, making them especially well-suited for pediatric patients and combination therapy strategies. This innovation addresses a critical therapeutic gap for patients who are unresponsive to or unable to tolerate existing treatments in the clinic.
Clinical and commercial potential by improving clinical outcomes
Clinically, this RET-targeting antibody has the potential to significantly improve outcomes for patients with RET-expressing tumors by providing a safer and more durable alternative to existing treatments. Its application may be especially impactful in pediatric oncology, where long-term safety is a critical concern. From a commercial perspective, the antibody represents a novel immunotherapeutic platform with broad potential across multiple cancer types. This RET-targeting antibody can be used as a monotherapy or in combination with other treatment which increases the therapy opportunities for expanded indications.Who should partner with us?
We are actively seeking strategic partners to help the advancement of this innovation by bringing this RET-targeting antibody to clinical applications and commercialization. Ideal collaborators and partners include:
- Biotechnology and pharmaceutical companies with expertise in antibody engineering, oncology drug development and immunotherapy
- Organizations with capabilities in translational research, GMP antibody manufacturing and early-phase clinical trials would be particularly valuable
- Academic or industry collaborators interested in combination therapy development or IND-enabling studies
Funding and future outlook
The development of this RET-targeting antibody has received Children’s National internal funding and institutional support for early-stage development, including proof-of-concept validation and in vivo efficacy studies. We are actively pursuing additional funding through NIH grants and strategic partnerships to support IND-enabling activities and clinical translation. We welcome interest from investors and collaborators who share our commitment to advancing innovative immunotherapies for cancer.
Contact: Haiyin Chang
Related News
-

Evolving curative therapies for pediatric non-malignant disorders
February 13, 2026 -
In the News: Why this clinical trial is offering some young cancer patients hope
January 09, 2026 -
New insights into genetics of childhood brain and spinal tumors
November 25, 2025