Muscle Biology and Diseases
Cell Biology of Myogenesis, Muscle Repair, Regeneration and Aging
The Center for Genetic Medicine Muscle Group focuses on understanding the cell biology of muscle and degenerative diseases. Research focus areas include muscular dystrophy, membrane trafficking, genetic diseases of muscle such as Duchenne muscular dystrophy, heart failure, steroid signaling/gene expression and microRNA regulation.
- Mitochondria as a Local Signaling Hub
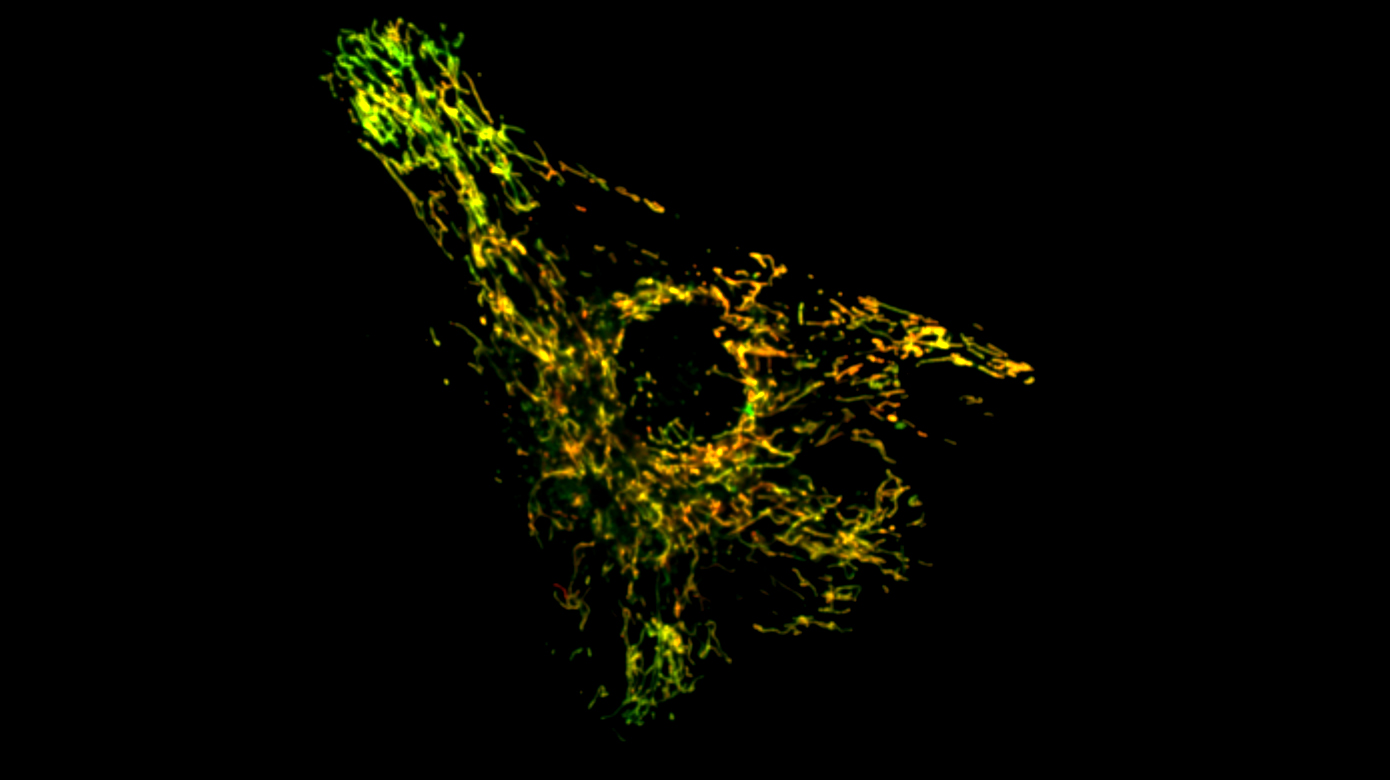
A serendipitous discovery that mitochondrial proteins accumulate at the injured cell membrane and mitochondria in muscle fiber can accumulate at the site of injury, led us to studies that have uncovered the requirement of mitochondria in repairing cell membrane injury. We find that calcium that enters the injured cell is taken up by mitochondria in a regulated manner which allows controlled production of reactive oxygen species (ROS). This ROS locally activates the reorganization of actin cytoskeleton to enable closure of the cellular wound. Despite being organized in a cell-wide network, mitochondria can act locally by controlled mitochondrial fission at the site of injury. We study how mutations that alter mitochondrial calcium uptake, ROS production or fission, compromise cell repair and lead to muscle diseases. Injury-mediated regulation of these processes in healthy cells, their downstream effectors and the role of mitochondrial interactions with other organelles in controlling this repair mechanism are some of the other open questions.
Our Team
Investigation of Antisense Oligonucleotide Delivery and Pharmacokinetics
The use of splice-switching antisense oligonucleotides (ASO) for genetic diseases like DMD has moved from the bench to the clinic. Despite this recent success, efficacy of ASO delivery has remained far from optimal, limiting the extent of clinical benefit of ASO-based therapies for DMD and other diseases. The laboratory of James Novak, PhD, together with the laboratories of Terence Partridge, PhD, and Jyoti Jaiswal, PhD, has recently identified tissue inflammation as an unexpected ally for overcoming this constraint. Working to understand the mechanism of ASO delivery in DMD, the team discovered that inflammatory cells function as an intramuscular reservoir that locally stores the intravenously-administered ASO in regions of muscle necrosis and repair (Novak et al. 2017). This finding points to a potentially novel strategy for systemic ASO delivery, involving the use of inflammatory cells as a Trojan horse. Such an approach would have the benefit not only of enhancing tissue-specific delivery of ASO, but also of reducing the impact of their rapid clearance from circulation. The team is also investigating the following:
- ASO delivery strategies for multi-exon skipping with the DMD exon 45-55 ‘hot spot’ region
- Mechanisms regulating the delivery of cell-penetrating peptide (CPP) antisense agents
- Long-term consequences of chronic morpholino exposure to muscle and kidneys
- Surrogate biomarkers as predictive outcome measures of morpholino-induced exon skipping at distinct periods of growth or heightened pathology in mouse models of the disease.
This research is supported by a NIH U54 grant on pediatric pharmacology, Parent Project Muscular Dystrophy and Early Stage Investigator Development Grants awarded to Dr. Novak through the M.D.A., Duchenne Parent Project – Netherlands and Foundation to Eradicate Duchenne.
Our Team
Pathophysiological Mechanisms and Therapies for Muscular Dystrophies
The team uses omics approaches to dissect disease mechanisms and identify circulating biomarkers for muscular dystrophies and autoimmune muscle disorders. Among the diseases studied, the lab has been most interested in facioscapulohumeral muscular dystrophy (FSHD) which is caused by both genetic and epigenetic mechanisms. After the double homebox protein 4 (DUX4) was identified as the causative gene of FSHD, the team has been studying molecular mechanisms that regulate DUX4.
Recently, the scientists shifted research focus to therapeutic development and preparation for clinical trials. In collaboration with Jean Mah, MD, and the Cooperative International Neuromuscular Research Group (CINRG), the lab conducted a natural history study of early onset FSHD and collected blood samples for biomarker studies using proteomes profiling and RNA-seq. For therapeutic development, the lab is investigating antisense oligonucleotide strategies for knocking down DUX4 in muscle cells using both cell and mouse models. A provisional patent has been filed for one of the compounds which are under investigation. Accurate and fast diagnosis of disease conditions is critical for successful disease interventions. FSHD is caused by contraction of 3.3 kb D4Z4 repeat, which posed technical challenges to using conventional molecular tools for molecular diagnosis. The team is currently developing a long-read sequencing assay for diagnosis and research using Nanopore MinION.
Our Team
Development of Improved Treatments for Muscle Diseases
Our goal is to develop next generation treatments and biomarkers for diseases in children. By targeting the underlying molecular biology of muscle diseases, the team believes it can help a broad array of human illnesses. For example, the lab is currently dissecting the molecular signaling pathways of steroids in DMD. This helped develop a new anti-inflammatory drug named Vamorolone to the point that it is now in Phase 2 clinical trials for DMD. Early work indicates this drug has the potential to replace prednisone as the standard of care for DMD, by providing a drug that is more effective while also being much safer. Ultimately, this can greatly improve treatment of DMD as well as a number of other diseases with shared molecular biology.
Improving Heart Failure and Fibrosis in Children
Heart failure is a leading cause of death in DMD, and is a feature of several other genetic muscle diseases. Currently, Marshall Hogarth, PhD, and Christopher Spurney, MD, are testing a new anti-inflammatory drug, Vamorolone, for children with DMD. They find that this drug also possesses a new property that improves heart failure and fibrosis. By acting as a mineralocorticoid receptor antagonist, Vamorolone effectively protects hearts and improves blood pressure in animal models. These data are consistent with clinical findings that another mineralocorticoid antagonist (eplerenone) improves heart failure in DMD patients. Moving forward, they will further investigate this new drug mechanism, determine the potential of vamorolone to treat heart failure in other diseases and identify blood-based biomarkers that predict heart health and the need for interventions.
Our Team
Using MicroRNA to Improve Treatment of Childhood Diseases and Chronic Inflammation
A new area of research, microRNA, is emerging as an important new source of biomarkers and therapeutic targets. We’ve found that a set of microRNAs are increased during states of inflammation, and conserved across several diseases featuring inflammation. By measuring levels of these microRNAs in blood, we find we can develop new minimally invasive measures that predict disease and drug response in children. Additionally, these same microRNAs are conserved in animal models of disease; we find that they can help guide and improve decisions on when to move a drug from preclinical animal testing into human clinical trials. Moving forward, we are developing next generation drugs that target these same microRNAs with high specificity in hopes to produce improved treatments for chronic inflammation. Our current focus is on DMD and inflammatory bowel disease, though we have supportive data on myositis and BMD as well. These microRNA studies are helping to develop new therapies, as well as new methods to track the effective treatment of patients.
Clinical Trials and Cooperative International Neuromuscular Research Group (CINRG)
The Cooperative International Neuromuscular Research Group (CINRG) is a consortium of medical and scientific investigators from academic and research centers who share the common goal of improving the lives of patients with neuromuscular disease and their families. The CINRG network joins together over 25 clinical and research sites from around the world to perform clinical studies in neuromuscular disorders. The group has successfully enrolled more than 1,200 study participants (predominantly children) into 18 studies to date. Heather Gordish-Dressman, PhD, serves as the principal investigator (PI) at Children’s National for both the CINRG Duchenne and Becker Natural History Studies.
The Duchenne Natural History Study (DNHS), chaired by CINRG PI Craig McDonald, MD, of UC-Davis, has completed funding through a combination of government (National Institute on Disability and Rehabilitation Research at NIH and DOD), foundation (Parent Project Muscular Dystrophy) and industry partner grants. It is the largest natural history study of DMD to date, with a wealth of data that provide natural history controls for both the design of industry trials and the interpretation of clinical trial data for many clinical and biochemical endpoints. The value of these data is recognized in the muscular dystrophy research community globally, resulting in increased interest in confidential access to the data. Dr. Gordish-Dressman continues this effort as lead statistician and has authored several publications, including a seminal manuscript published by the Lancet in November 2017.
The Becker natural history study is led by PI Paula Clemens, MD, in Pittsburgh. The study has reached its enrollment goals and is collecting long-term data on participants with Becker muscular dystrophy. The CoQ-10/Lisinopril clinical trial, funded by the DOD and led by Dr. Clemens, is continuing with long-term follow-up of participants. Dr. Gordish-Dressman is currently analyzing data from both of these important studies.
Focus Areas
- Membrane trafficking
- Muscular dystrophy
- Facioscapulohumeral muscular dystrophy (FSHD)
- Developmental disability
- Host-pathogen interaction
- Drug development
- Heart failure
- MicroRNA to improve treatment in children
- Skeletal and cardiac muscle disease pathogenesis
- Therapeutic targets for muscular dystrophies
Faculty with Interests in Muscle Biology and Diseases

Jyoti K. Jaiswal, MSc, PhD
- Senior Investigator
- Director, Cell and Tissue Microscopy Core